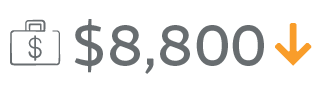Reduction in the incidence of SSIs
The PICO System reduced the risk of surgical site complications (SSCs) in patients undergoing primary hip and knee arthroplasty, compared to standard care. (p=0.06)3
Watch a live knee OR application of the PICO System, to see how quick and easy it is to apply.
A patient's perspective-Kurt's story
One patient's journey to recovery after hip surgery with the PICO System.
How does PICO therapy work?
Only PICO sNPWT dressings have AIRLOCK* Technology, to help promote effective outcomes.5-8
Application of the PICO System after knee surgery
-
Gilchrist B, Robinson M, Jaimes H. Performance, safety, and efficacy of a single use negative pressure wound therapy system for surgically closed incision sites and skin grafts: A prospective multi-centre follow-up study. Paper presented at: SAWC; 2020; Virtual.
-
Hurd T, Trueman P, Rossington A. Use of a Portable, Single-use Negative Pressure Wound Therapy Device in Home Care Patients with Low to Moderately Exuding Wounds: A Case Series. Ostomy Wound Manage. 2014;60(3):30-36.
-
Karlakki SL, Hamad AK, Whittall C, et al. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: A randomised controlled trial. Bone Joint Res. 2016;5(8):328-337.
-
Nherera LM, Trueman P, Karlakki SL. Cost-effectiveness analysis of single-use negative pressure wound therapy dressings (sNPWT) to reduce surgical site complications (SSC) in routine primary hip and knee replacements. Wound Repair Regen. 2017;25(3):474-482.
-
Saunders C, Nherera LM, Horner A, Trueman P. Single-Use negative-pressure wound therapy versus conventional dressings for closed surgical incisions: systematic literature review and meta-analysis. BJS Open. 2021;0(0):1 - 8.
*n=209, p=0.002. High-risk patients deemed to be those with BMI >35 or ASA >3